FDA’s eCTD Mandate: Are You Ready?
When FDA posted their eCTD mandate last year, it was more of a whisper than a bang. It certainly did not wreak havoc in the industry; after all, we were all warned that the time would come sooner rather than later.
The deadlines are quite clear:
After May 5, 2017
The following submission types must be filed in eCTD format:
- NDAs
- ANDAs
- BLAs
- MFs
After May 5, 2018
The following submission types must be filed in eCTA format:
- Commercial INDs
Source: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/
FormsSubmissionRequirements/ElectronicSubmissions/ucm153574.htm
*Submissions not filed in eCTD format will not be filed or received.
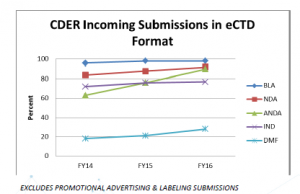
Statistics show that the majority of incoming submissions are already in eCTD format:
Source: Electronic Submissions Update presentation at RAPS 2016: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/
FormsSubmissionRequirements/ElectronicSubmissions/UCM522105.pdf
Roughly 90 percent of the industry is already filing in eCTD format (DMF filings at least are on the rise) to FDA. For those of you still holding out, FDA’s eCTD mandate is now forcing your hand to make the switch. If you’re currently filing paper submissions for IND, you have a year to consider the following:
1. To host or not to host?
eCTD software is expensive. There exist different cost structures, depending on your decision to host the software in-house or access the software via a remote host. Hosting eCTD software in-house entails a battery of qualification and validation steps, and can be practical if you already have an IT group with relevant expertise, or can enlist one to manage the installation. If you’re not in possession of a Computer Science degree, the amount of work and length of time (and the terminology) might be daunting. Increasingly, eCTD software providers are making cloud based solutions available, which allow you to access the eCTD software remotely. Your eCTD software vendor can then perform most of the installation and qualification steps, with minimal qualification steps required by you as the end user.
2. Submission compilation – keep in-house or outsource?
This is another key point for consideration. What would be the utility of having (expensive) eCTD software if you don’t know how to use it to maximize the potential, or compile a submission that meets FDA’s requirements? Like it or not, FDA has specifications for your PDF files, metadata for submission types that need to be entered correctly or else you run the risk of your submission being rejected. There are also PDF tools that can facilitate PDF processing at the click of a mouse. All of these activities require trained professionals and standard operating procedures in order to ensure a consistent and high quality product.
3. Electronic Submissions Gateway (ESG) or Courier?
eCTD and ESG go hand in hand. Submissions under 10GB should be filed through the Gateway. Any submissions exceeding 10GB can also be uploaded to the ESG or can be submitted on appropriate physical media and sent by courier to FDA. You can dispatch your eCTD immediately through ESG once your submission has your internal stamp of approval (no printing, and no more paper cuts!). Using the ESG also provides an electronic receipt and acknowledgement files, reducing post-filing anxiety (did the courier arrive at FDA? In one piece?), and enabling quicker notification to your submission team that FDA has your submission for review. However, in order to use the ESG, you must first be qualified by FDA and undergo a sample submission review process and electronic certificate exchange.
If all of the above requirements to transition to eCTD seem costly, lengthy to implement, and worrisome, Intrinsik can help!
We have filed over 300 IND sequences over the past 12 months (including clients who have already switched from paper to eCTD), and have a full in-house Regulatory Operations department with specialists in filing eCTD submissions.
For more information on how Intrinsik can help your company, please contact .
Intrinsik Blogger:
Anisah Bipatnath, M.Sc.