Acceptable Intake Limits Now More Clearly Defined for Nitrosamine Impurities and Nitrosamine Drug Substance-Related Impurities (NDSRIs)
Nitrosamines are a class of compounds with the chemical structure shown in Figure 1. In the International Council for Harmonisation (ICH) M7(R1) Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk guidance document (ICH 2018), nitrosamine compounds were identified as a “cohort of concern” based on potent genotoxicity observed in several animal species and some compounds (e.g., N-nitrosodimethylamine [NDMA] and N-nitrosodibutylamine [NDBA]) being classified as probable or possible human carcinogens by the International Agency for Research on Cancer (IARC) (FDA 2020). Therefore, acceptable intake (AI) limits for this “cohort of concern” can be estimated but would likely be significantly lower than AI limits defined for compounds of negligible carcinogenic/toxic risk (ICH 2018). If limited carcinogenicity data exists for the identified nitrosamine, the carcinogenicity data from closely related structures can be relied upon to justify an AI limit for the drug product.
Figure 1 Chemical Structure of Nitrosamines
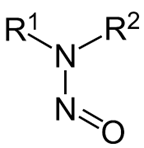
Figure above shows a nitroso group bonded to an amine, with other compound constituents (R1 and R2) surrounding this functional group.
In June 2018, the United States (US) Food and Drug Administration (FDA) was informed of the presence of a nitrosamine (i.e., NDMA) in the angiotensin II receptor blocker (ARB), valsartan (Gottlieb 2018; FDA 2020). Numerous lots of valsartan and a few other ARB drug products from different manufacturers contained unacceptable levels of nitrosamines. A recall of the affected batches resulted in a drug shortage for some of the ARBs affected. Based on ICH M7(R1) and the findings with valsartan, all drug products currently available on the Canadian, US, and European Union (EU) markets were evaluated for nitrosamine impurities (EMA 2023; FDA 2020; Health Canada 2023). Since then, a number of drug products were found to contain unacceptable levels of nitrosamine drug substance-related impurities (NDSRIs; FDA 2020, FDA 2023). NDSRIs are a class of nitrosamines which share structural similarity to the drug substance, as they can be formed during the synthesis of the drug substance where precursor amines (e.g., primary, or secondary amines) from earlier steps react with a nitrosating agent (e.g., nitrite) introduced in subsequent steps. However, they may also form during manufacture and storage of the drug product. For example, nitrite impurities present in excipients at parts-per-million amounts can act as nitrosating agents in the presence of a precursor amine.
In response to the identified risk associated with nitrosamine impurities (i.e., both NDSRIs and other nitrosamines) found in a number of drug products, both the FDA and Health Canada released specific guidance documents (FDA 2023; Health Canada 2023) to help define the AI limits of nitrosamine-containing compounds commonly found in drug products. The agencies have also agreed upon a standardized approach, the Cancer Potency Categorization Approach (CPCA), based on the unique structure of the NDSRI to assign AI limits to any new or existing NDSRIs. More clearly defined AI limits for these nitrosamine and NDSRI compounds essentially sets a threshold for all drug products to meet on the Canadian, US, and EU markets, rather than relying on an AI limit justified by each Sponsor which may vary across drug products. This approach taken by the Canadian, US, and EU can therefore ensure better safety for drug products available on their respective markets. In the EU, FDA and Canadian guidance, consistent AI limits are provided for a large number of nitrosamines and NDSRIs, primarily based on the CPCA approach. Importantly, this same methodology can be used for NDSRIs that are not currently in the agency databases, and for which AI limits will need to be determined to establish a safety margin for a Sponsor’s drug product. Intrinsik can help with CPCA calculations and alternative approaches to establishing AI limits.
References
Gottlieb S. FDA Statement on FDA’s ongoing investigation into valsartan impurities and recalls and an update on FDA’s current findings. FDA statement, August 2018. https://www.fda.gov/news-events/press-announcements/fda-statement-fdas-ongoing-investigation-valsartan-impurities-and-recalls-and-update-fdas-current
Health Canada. October 2023. Guidance on nitrosamine impurities in medications: Evaluating and managing the risks of N-nitrosamine impurities in human pharmaceutical, biological and radiopharmaceutical products.
International Conference on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use (ICH). March 2018. Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk M7(R1).
US Food and Drug Administration (FDA). September 2020. Guidance for Industry Control of Nitrosamine Impurities in Human Drugs. Center for Drug Evaluation and Research (CDER).
US Food and Drug Administration (FDA). August 2023. Guidance for Industry Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs). Center for Drug Evaluation and Research (CDER).
For more information on how Intrinsik can help your company, please contact .
Intrinsik Blogger: Dr. Danielle Drake

